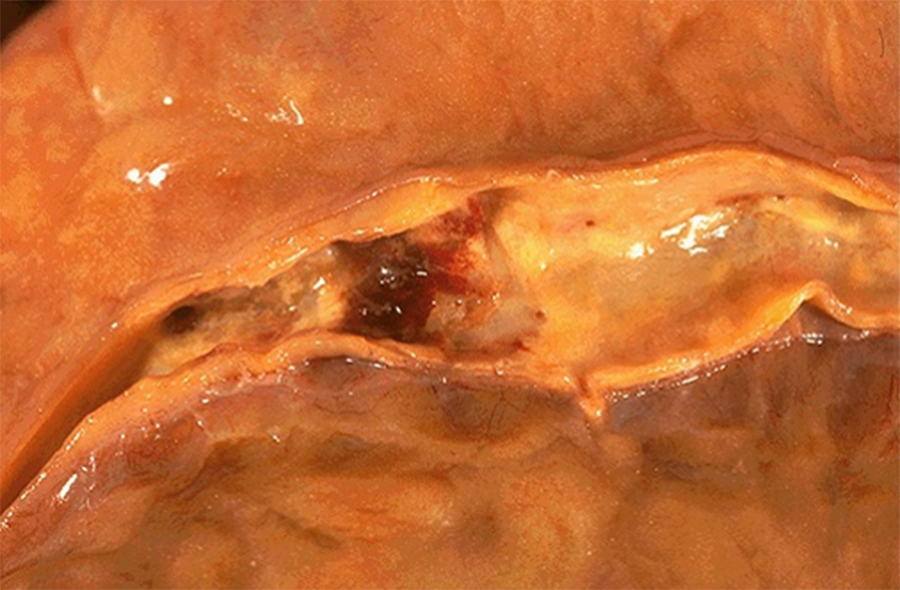
Image 1: The coronary arteries are dissected from the epicardial surface and breadloafed. The patient's right coronary artery is seen in the gross image. Similar changes were found in the other coronary arteries.
Question: What process is occurring?
Question: What approximate percentage of (cross-sectional area) narrowing is necessary to produce ischemic symptoms with mild exercise (manifesting as angina)?
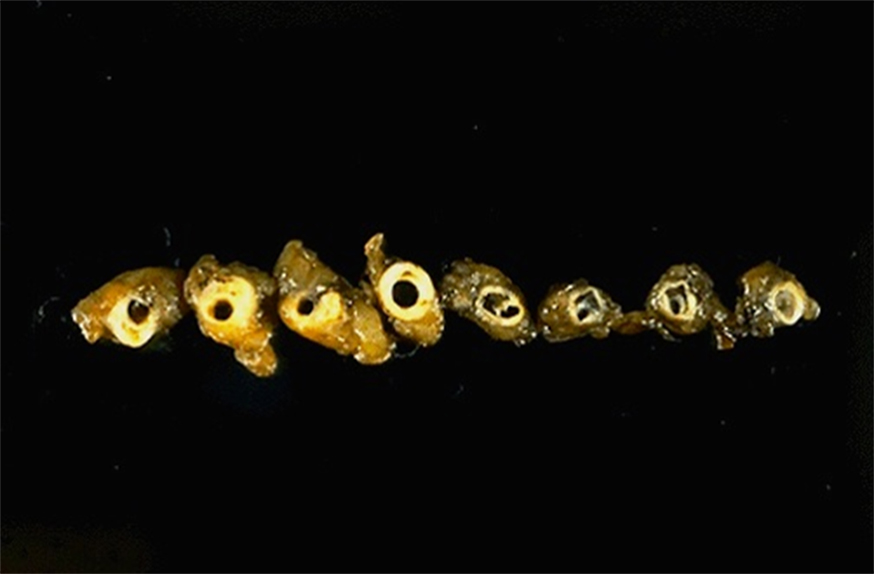
Image 2: This slide represents five cross-sections through the breadloafed coronary artery. At low power, thickened arterial walls with narrowed lumens can be appreciated. The luminal narrowing can be concentric or eccentric, depending upon how atherosclerotic plaque (AKA: atheroma) development affects the particular region. On a couple of cross-sections, smaller arterial branches can be seen adjacent to the coronary artery proper.
On high power and examining the left-most cross-section, classic features of atherosclerosis can be identified. As atherosclerosis is currently viewed, chronic endothelial injury to the wall for any reason (hyperlipidemia, smoking, hypertension, immune reactions, etc.) leads to both 1) an altered inflammatory response and 2) an attempt at wound healing. Chronic inflammatory cells (lymphocytes and macrophages) can be seen residing in the wall, and are drawn to the area due to on-going endothelial injury. Lipoproteins will begin to accumulate and eventually will be ingested by macrophages, forming foamy macrophages which can be seen around the central core of cholesterol and cholesterol esters; during tissue processing, cholesterol is effectively washed away, leaving only the angular or needle-shaped spaces of its former distribution. Over time, macrophages and inflammatory-recruiting factor release lead to smooth muscle cell recruitment and proliferation. Both smooth muscle cells and macrophages continue to uptake lipid, but as is this slide, much of it collects extracellularly within the central core. Smooth muscle cells begin to produce collagen and other extracellular matrix proteins, which will eventually surround the developing process, producing the fibrofatty atheroma that can be seen in this slide. Overlying the central lipid core, the fibrous cap that faces the lumen helps to stabilize the atheroma.
Other cross-sections show interesting features as well. Endothelial inflammation is nicely demonstrated in the upper right cross-section, and focal periadventitial inflammation can be found around the center cross-section. The center cross-section also shows dystrophic calcification within the atheroma, likely due to the death of differing cells types. Finally, the uppermost cross-section shows the intensely eosinophilic appearance of mature collagen, sometimes prominently seen at the periphery of an atheroma or as the predominant finding within any particular atheroma.
Image 3: Coronary artery disease leading to chronic myocardial ischemia can be subclinical (i.e., not clinically apparent), however, gross and microscopic features will provide the most definitive information. Gross features of our patient's heart are unremarkable, including on weight, shape and on cut surface.
Microscopically though, examination of our patient's left ventricle gives additional information regarding the extent of her coronary artery disease. At low power, and particularly in the upper half of the slide, the intense eosinophilia imparted by the cardiac myocytes is punctuated by paler (eosinophilic) regions and foci of intense basophilia. On higher power, the myocytes appear unremarkable and note lipofuscin present within the cytoplasm of many myocytes - an essentially normal feature due to aging. The patchy, paler regions consist of mature collagen surrounding small vessels, replacing/displacing variable regions of myocytes, and even surrounding some individual myocytes. This collagen is indicative of ischemia sometime in the past resulting in myocyte death and replacement by connective tissue, due to normal wound healing. Because the ischemia is occurring chronically and often subclinically, variable myocyte irreversible injury leads to patchy myocyte death and subsequently patchy replacement by collagen.
Question: How might this histologic appearance differ with an acute ischemic episode that occurred at one point in time (i.e., a clinically apparent myocardial infarction)?
The basophilic material can be appreciated as dystrophic calcification variably developing within the regions of fibrosis. Recall that calcium influx occurs as cells undergo injury, and following cell death, can collect or pool as an extracellular deposits. These calcium deposits often appear coarse, chunky, and intensely basophilic or magenta on typical H/E stains.
Question: What cardiac-related laboratory parameter(s) could have shown elevation sometime in our patient's past?
Image 4: Dissection into the mesentery revealed the following within a large artery.
Question: What major cell types comprise the mesentery?
Question: What two major pathologic processes are depicted in this image?